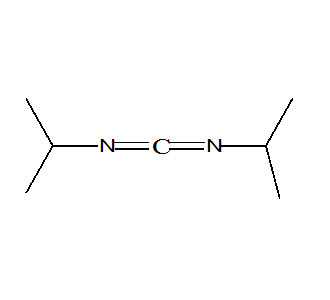
Diisopropyl Carbodiimide (DIC), identified chemically as N,N′-Diisopropylcarbodiimide, is a widely used coupling and dehydrating agent in pharmaceutical synthesis, peptide chemistry, polymer science, and fine organic transformations. When sourcing this reagent, choosing reliable Diisopropyl Carbodiimide Manufacturers In India is essential, as purity, storage stability, and batch consistency directly influence reaction yield and reproducibility. Since DIC is frequently used in the formation of amide bonds and activation of carboxyl functional groups, the quality of the reagent plays a critical role in controlling by-product formation, stereoselectivity, and end-product purity.
Chemical Identity and Structural Features
Diisopropyl Carbodiimide belongs to the carbodiimide family, characterized by the functional group –N=C=N–. This central carbodiimide moiety performs the key role of activating carboxyl groups for nucleophilic substitution reactions.
Key Molecular Details:
- Chemical Formula: C₇H₁₄N₂
- Functional Group: Carbodiimide
- Primary Role: Activation of carboxylic acids for amide, ester, and peptide bond formation
- Physical State: Colorless to pale yellow liquid
- Stability: Sensitive to moisture and acidic environments
Its compact molecular size and sterically hindered isopropyl groups make DIC particularly useful when minimizing side reactions and urea by-product polymerization is important.
Applications in Pharmaceuticals and API Synthesis
The pharmaceutical industry relies heavily on amide bond formation, and DIC provides an efficient route to achieve this without aggressive reaction conditions. It is widely used in:
1. API and Intermediate Synthesis
In multi-step synthetic routes where amide bonds occur repeatedly, DIC ensures:
- Controlled activation of acids
- Minimal racemization when chiral centers are present
- Formation of stable intermediates suitable for downstream modifications
Thus, DIC supports the creation of therapeutic molecules, enzyme inhibitors, and small-molecule drug candidates.
2. Synthesis of Ureas and Related Nitrogen Functionalities
DIC can react with amines and carbonyl compounds to produce urea and carbamate-containing structures, which appear in:
- CNS active molecules
- Antiviral intermediates
- Antiinflammatory drug scaffolds
Because these transformations occur under relatively mild conditions, DIC is well-suited for sensitive or sterically complex substrates.
Crucial Role in Peptide Chemistry
Peptide synthesis requires precise amide bond formation between amino acids, often under conditions that prevent racemization. DIC is a recognized reagent in both solid-phase and solution-phase peptide synthesis (SPPS).
Advantages of DIC in Peptide Assembly
- Low Racemization: Protects chiral integrity of amino acids
- Cleaner Reaction Profiles: Generates minimal side products when paired with additives such as HOBt or Oxyma
- Compatibility with Protected Peptide Systems: Works well with Fmoc and Boc methodology
This makes DIC one of the most important reagents in modern biopharmaceutical and peptide-based therapeutic development.
Applications in Polymer and Material Science
In polymer research and industrial material modification, DIC is used as:
- A dehydrating reagent for polymer end-group functionalization
- A chain-extending agent to modify molecular weight
- A crosslinking facilitator in urethane and epoxy system development
These transformations are significant in producing:
- High-performance engineering plastics
- Biomedical hydrogels
- Polymer-based drug delivery systems
Because DIC promotes controlled functionalization, it enhances mechanical strength, durability, and reactivity of polymer architectures.
Sourcing Considerations, Specifications & Handling
Partnering with experienced and quality-focused Diisopropyl Carbodiimide Manufacturers In India ensures reliable sourcing, consistent purity, safe handling, and long-term production continuity across laboratory and manufacturing scales.
To ensure reliable performance, several quality and sourcing parameters must be evaluated:
- Purity: High assay purity limits formation of diisopropyl urea residues.
- Moisture Sensitivity: Must be stored in airtight containers to maintain stability.
- Color Stability: Fresh batches are clear; discoloration can indicate decomposition.
- Packaging: Glass or chemical-resistant polymer containers are recommended.
- Regulatory Documents: COA, MSDS, ISO, and GMP compliance (if used in pharmaceutical workflows).
Storage should be under dry, cool, and inert conditions, ideally nitrogen-protected, to prevent premature hydrolysis.
Handling should always be conducted in a well-ventilated fume hood, using gloves and eye protection.
Safety Notes
- DIC can cause skin and respiratory irritation if handled improperly.
- Avoid contact with strong acids, bases, and oxidizers.
- Waste should be disposed of as hazardous organic material in accordance with regulatory protocols.
Proper handling not only protects personnel but ensures reactive efficiency and reproducibility in synthesis workflows.
Conclusion
Diisopropyl Carbodiimide is an essential reagent in pharmaceutical synthesis, peptide chemistry, fine organic synthesis, and polymer modification due to its efficiency in forming amide bonds and activating carboxyl groups under controlled conditions. Its role in producing high-purity peptides, functionalized drug intermediates, and advanced polymeric materials highlights its industrial importance.
Other Recommendations: CAS NO 762-04-9 Manufacturers